Author Affiliations
1. Laboratoire d'Ecologie, Systématique
et Evolution, Université Paris-Sud
CNRS UMR 8079, Bâtiment 362,
91405 Orsay Cedex, France
2. Department of Biological Sciences,
University of South Carolina
Columbia, SC 29208, USA
Author for correspondence
anders.moller@u-psud.fr
Abstract
Effects of low-level
radiation on abundance of animals are poorly known. We conducted standardized
point counts and line transects of bumble-bees, butterflies, grasshoppers,
dragonflies and spider webs at forest sites around Chernobyl differing
in background radiation by over four orders of magnitude. Abundance of
invertebrates decreased with increasing radiation, even after controlling
for factors such as soil type, habitat and height of vegetation. These
effects were stronger when comparing plots differing in radiation within
rather than among sites, implying that the ecological effects of radiation
from Chernobyl on animals are greater than previously assumed.
1. Introduction
The ecological consequences
of radiation from Chernobyl are poorly known (Møller
& Mousseau 2006). Surprisingly, there are few data on the abundance
of animals in relation to radiation, and there have been no efforts to
extensively census invertebrates in relation to radiation. The
few studies published are mainly based on sampling in a few locations,
making it difficult to generalize about the effects of radiation when compared
with other factors (Krivolutsky & Pokarshevsky 1992;
Sokolov et al. 1994; Maksimova 2002; Jackson et al. 2005).
The objectives of this
study were to assess the abundance of invertebrate taxa in relation to
background radiation. These were insect pollinators (bumble-bees and butterflies),
an important taxon of herbivores (grasshoppers) and predators (dragonflies:
and spiders). We conducted two kinds of census: point counts covering more
than 700 sites during 3 years and line transects. This is by far the most
extensive dataset on the abundance of animals from Chernobyl. Abundance
can be affected by environmental factors other than radiation, and, therefore,
we controlled statistically for potentially confounding variables that
could affect the relationship between abundance and level of radiation.
Line transects provided a powerful within-site experimental design controlling
for many confounding variables because neighbouring sites that differ in
the level of radiation by definition are similar in most respects, including
soil type, habitat and other features.
2. Material and methods
A.P.M. conducted 731
standard point counts over the 3 years, late May–early June 2006–2008,
within forested areas around Chernobyl, each count lasting 5?min during
which all birds seen or heard, bumble-bees, butterflies, spider webs and
dragonflies (dragonflies were only censused in 2008) were recorded (Møller
1983; Bibby et al. 2005). Points were located at 75?m intervals. Grasshoppers
were censused during early September 2007 at 374 points.
We conducted four line
transects at 17 sites during early July 2008 within and just outside the
southern border of the exclusion zone. At each site, we pre-selected plots
with high and low radiation [high radiation: 0.60 mGy.h-1
(s.e.=1.29), low radiation: 0.38 mGy.h-1
(1.29)]. At each plot, A.P.M. conducted two line transects along roads
in opposite directions, each of 50 m. Walking speed was slow, each transect
lasting 10 min. Half an hour later, the same line transects were conducted
once more without reference to the first census results.
Invertebrate abundance
estimates can be affected by habitat (agricultural habitats with grassland
or shrub, deciduous forest or coniferous forest estimated to the nearest
10% of ground coverage within 50 m from each of the observation points),
maximum height of trees estimated to the nearest 5 m, soil type (loam/clay
or sand), presence or absence of open water within 50 m from each point,
cloud cover at the start of each point count (to the nearest eighth), temperature
(range 12–28°C) and wind force (Beaufort, range 0–4). For each point
count, we recorded time of day (to the nearest minute). Because
the activity of many invertebrates is curvilinearly related to time of
day, with high levels of activity in the middle of the day (e.g. Barnes
et al. 2001), we also included time squared in the analyses.
Once having finished a 5 min
census, we measured a,
b
and g radiations two to three times at
ground level using a hand-held dosimeter (Model: Inspector, SE International,
Inc., Summertown, TN, USA). Cross-validation against data from Shestopalov
(1996) revealed a strong positive relationship (linear regression on
log–log transformed data: F=1546.49, d.f.=1,252, r2=0.86,
p<0.0001,
slope (s.e.)=1.28 (0.10)), suggesting reliability of our estimates.
Abundance of invertebrates
and radiation level were log transformed, while coverage with farmland
and deciduous forest was square-root arcsine transformed. We
developed best-fit models to assess the relationship between invertebrate
abundance (dependent variable) and radiation, after the inclusion of potentially
confounding environmental variables, as implemented in JMP (SAS
Institute Inc. 2000). Model selection was based
on Akaike's information criterion (AIC), using the criterion of delta AIC<2.00
for exclusion of variables (Burnham & Anderson 2002).
We analysed line transect data by using abundance as the response variable
and site, level of radiation and their interaction as predictor variables.
Frequency distributions of invertebrate counts were skewed, with disproportionately
many zeros. However, we obtained similar results with Kendall rank-order
correlation and partial rank-order correlation (controlling for confounding
variables in table S2 in the electronic supplementary material).
3. Results
The abundance of invertebrates
decreased with increasing radiation (figure 1). We recorded 298
bumble-bees with Bombus terrestris accounting for 72.6% of all observations
(see table S1 in the electronic supplementary material). Abundance of bumble-bees
decreased between normal background radiation levels and the most contaminated
areas (figure 1a). In addition, there were effects of year, temperature,
wind and habitat on the abundance of bumble-bees (see table S2 in the electronic
supplementary material). We recorded 389 butterflies with Aporia crataegi
accounting for 36.6% (see table S3 in the electronic supplementary material).
Again, abundance decreased significantly between sites with level of contamination
(figure 1b). Abundance of butterflies was additionally explained
by time of day, wind and habitat (see table S2 in the electronic supplementary
material).
Figure 1
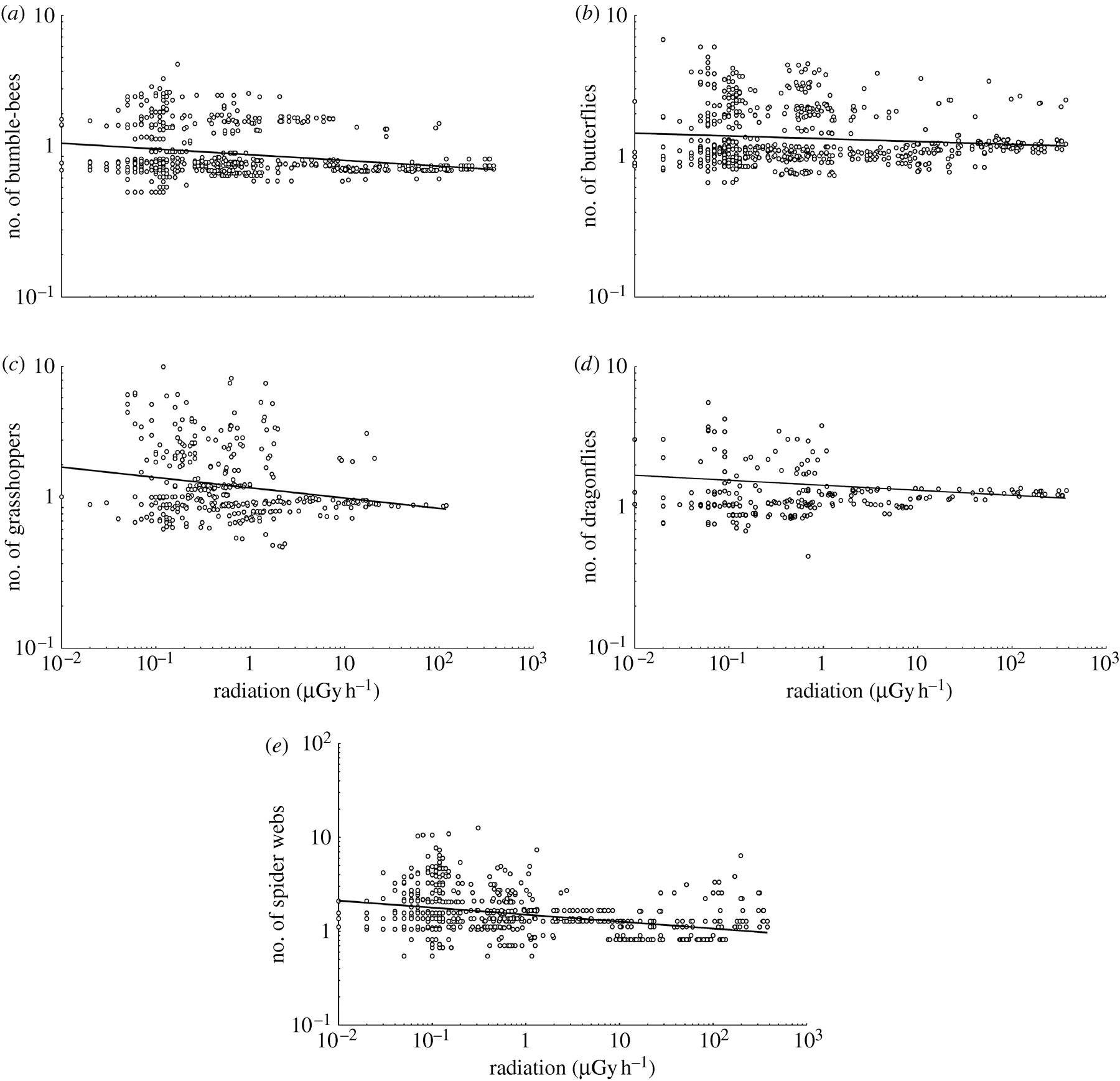
Abundance of (a) bumble-bees, (b) butterflies,
(c) grasshoppers, (d) dragonflies and (e) spider webs estimated during
point counts in 2006–2008 in relation to background radiation (?Gy?h?1)
for forested locations around Chernobyl, Ukraine and Belarus. (The lines
are the linear regression lines.)
We recorded 305 grasshoppers
during our point counts. Abundance decreased significantly with radiation
(figure 1c), with additional effects of date, time of day, temperature,
cloud cover and habitat (see table S2 in the electronic supplementary material).
The total number of dragonflies was 105 during point counts, with abundance
decreasing significantly with radiation (figure 1d), time of day
and habitat (see table 1D in the electronic supplementary material). Finally,
we recorded 775 spider webs, with abundance declining with radiation (figure
1e), year, temperature and wind (see table S2 in the electronic supplementary
material).
|
suite:
The census results from
repeated line transects were consistent (see table S4 in the electronic
supplementary material). All five invertebrate taxa
decreased significantly in abundance with increased radiation (figure
2), with intermediate to large effects (sensu Cohen 1988)
accounting for 14–38% of the variance (see table S4 in the electronic supplementary
material). There were also significant site effects, with the exception
of spider webs (see table S4 in the electronic supplementary material).
Grasshoppers and butterflies showed significant interactions between radiation
and site (see table S4 in the electronic supplementary material).
Figure 2
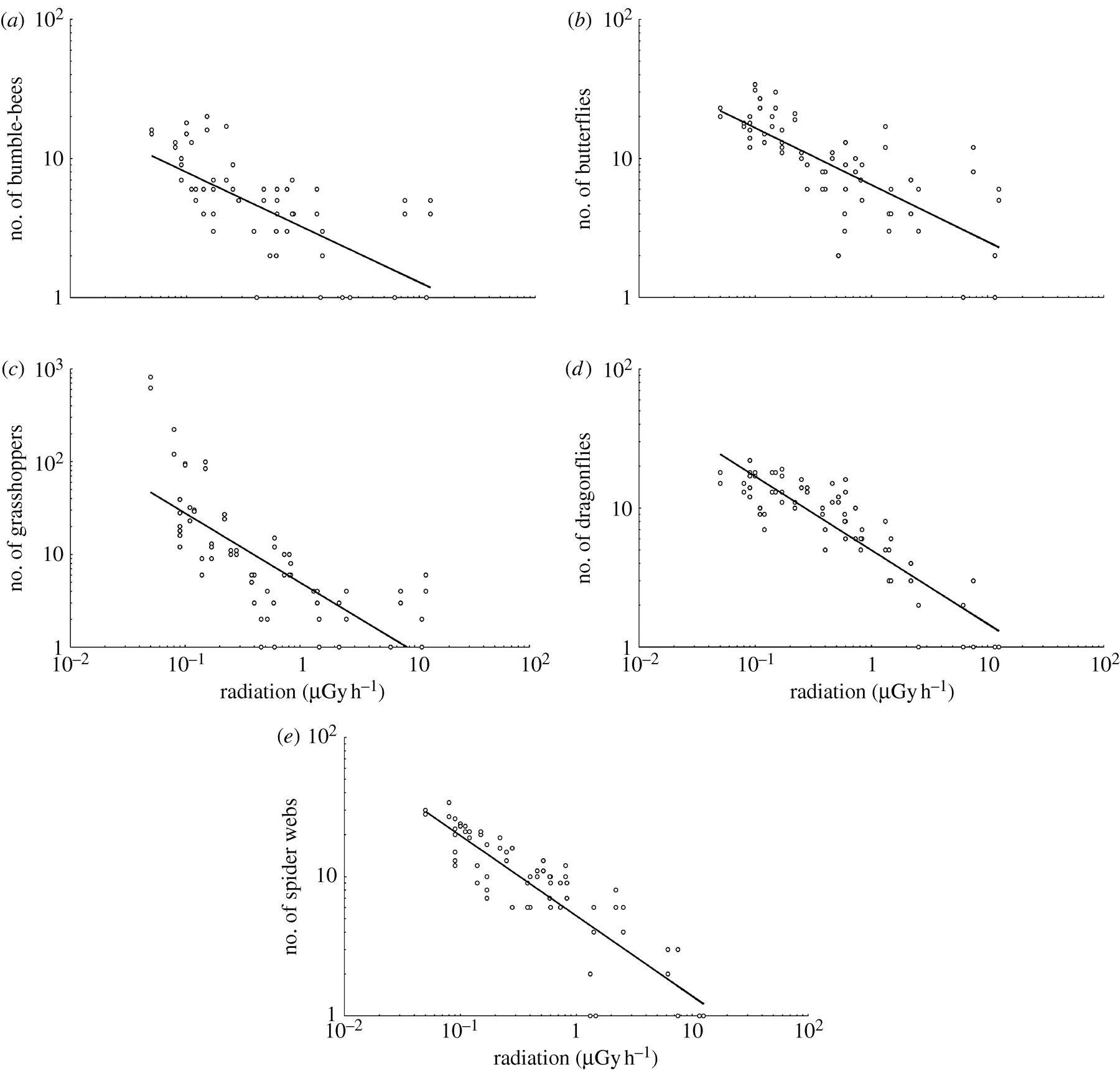
Abundance of (a) bumble-bees, (b) butterflies,
(c) grasshoppers, (d) dragonflies and (e) spider webs estimated during
line
transects in July 2008 in relation to background radiation (?Gy?h?1) around
Chernobyl, Ukraine and Belarus. (The lines are the linear regression lines.)
4. Discussion
We found declining abundance
of invertebrates with radiation near Chernobyl, especially in line transects
that controlled for confounding environmental variables through within-site
comparisons. The only comparable data for abundance
and diversity of birds in Chernobyl show similar patterns (Møller
& Mousseau 2007). The negative relationship extended to the range
0.01–1mGy.h-1 (figures 1 and
2),
suggesting that invertebrates are affected even at levels of contamination
below one hundred times normal levels.
Most radiation around Chernobyl
is currently in the topmost soil (Shestopalov 1996),
where most invertebrates live. For example, butterfly eggs, larvae or pupae
spend time in the soil layer or the vegetation just above. This could negatively
affect survival and fecundity, and hence abundance. Alternatively, indirect
effects of radiation on prey could potentially explain the reduced abundance
of spiders and dragonflies, but not the reduced abundance of bumble-bees,
butterflies and grasshoppers.
Point counts covered
many different sites, implying that factors other than radiation varied
among sites. Not surprisingly, we, on average, explained only 6% of the
variance. The second kind of census based on a within-site design better
controlled for confounding variables. On average, the relationship between
abundance and radiation explained 22% of the variance based on transects.
These results have implications
for ecosystems and overall ecosystem functioning. Reduced
abundance of pollinators generally affects plant fecundity and seed set,
when plant fecundity is pollen limited (Proctor 1996). Likewise,
spiders are important predators (Wise 1993), and reductions
in abundance can have important consequences for abundance of other invertebrate
taxa (e.g. Snyder & Wise 2001). Pollination
and predation are considered important ecosystem services (Costanza
et al. 1997), and disruption may affect the overall ecosystem functioning,
suggesting that the Chernobyl region and its surroundings is a perturbed
ecosystem.
Acknowledgements
We acknowledge help
from I. Chizhevsky, G. Milinevski, G. Rudolfsen, S. Rushkovsky and N. Saino.
We gratefully acknowledge support from US National Science Foundation,
Samuel Freeman Charitable Trust, CRDF, NATO CLG program, Fulbright Program
and National Geographic Society.
Footnotes
Received December 18, 2008.
Accepted February 12, 2009.
© 2009 The Royal Society
Notes:
1 Barnes R.S.K, Calow
P, Olive P.J.W, Golding D.W, Spicer J.J
The invertebrates. In Blackwell 2001 Malden,
MA:Blackwell
retour au texte
2 Bibby C.J, Hill D.A,
Burgess N.D, Mustoe S
Bird census techniques. In Academic Press 2005
London, UK:Academic Press
retour au texte
3 Burnham K.P, Anderson
D.R
Model selection and multimodel inference. In
Springer 2nd edn. 2002 New York, NY:Springer
retour au texte
4 Cohen J
Statistical power analysis for the behavioral
sciences. In Lawrence Erlbaum 2nd edn. 1988 Hillsdale, NJ:Lawrence Erlbaum
retour au texte
5 Costanza R, et al.
1997 The value of the world's ecosystem services
and natural capital. Nature. 387, 253–260. doi:10.1038/387253a0.
retour au texte
6 Jackson D, Copplestone
D, Stone D.M, Smith G.M
2005 Terrestrial invertebrate population studies
in the Chernobyl exclusion zone, Ukraine. Radioprotection. 40, S857–S863.
doi:10.1051/radiopro:2005s1-126.
retour au texte
7 Krivolutsky D.A, Pokarshevsky
I
1992 Effects of radioactive fallout on soil animals
populations in the 30?km zone of the Chernobyl atomic power plant. Sci.
Total Environ. 112, 69–77. doi:10.1016/0048-9697(92)90239-O.
retour au texte
8 Maksimova S 2002 The
effect of radioactive contamination caused by the Chernobyl nuclear accident
on Diplopoda communities. Acta Zool. Lituanica. 12, 90–94.
retour au texte
9 Møller A.P Methods
for monitoring bird populations in the Nordic countries. In Nordic Council
of Ministers 1983 Oslo, Norway:Nordic Council of Ministers
retour au texte
10 Møller A.P,
Mousseau T.A
2006 Biological consequences of Chernobyl: 20
years after the disaster. Trends Ecol. Evol. 21, 200–207. doi:10.1016/j.tree.2006.01.008.
retour au texte
11 Møller A.P,
Mousseau T.A
2007 Species richness and abundance of birds
in relation to radiation at Chernobyl. Biol. Lett. 3, 483–486. doi:10.1098/rsbl.2007.0226.
retour au texte
12 Proctor M The natural
history of pollination. In Timber Press 1996 Portland, OR:Timber Press
retour au texte
13 SAS Institute Inc.
JMP. In SAS Institute Inc 2000 Cary, NC:SAS Institute Inc
retour au texte
14 Shestopalov V.M Atlas
of Chernobyl exclusion zone. In Ukrainian Academy of Science 1996 Kiev,
Ukraine:Ukrainian Academy of ScienceAcademy of Science
retour au texte
15 Snyder W.E, Wise
D.H 2001 Contrasting trophic cascades generated by a community of generalist
predators. Ecology. 82, 1571–1583. doi:10.2307/2679801.
ISI
retour au texte
16 Sokolov V.E, Ryabov
I.N, Ryabtsev I.A, Kulikov A.O, Tichomirov F.A, Sheheglov A.I
1994 Effects of radioactive contamination on
the flora and fauna in the vicinity of Chernobyl nuclear power plant. Sov.
Sci. Rev. F Physiol. Gen. Biol. Rev. 8, 1–124.
retour au texte
17 Wise D.H Spiders
in ecological webs. In Cambridge University Press 1993 Cambridge, UK:Cambridge
University Press
retour au texte |

